|
HOME > Proposed research projects > 2012-2013: Proposed research projects 17
 2012-2013: Proposed research project 17Functional analysis of the tumor suppressor APC in epithelial tubulogenesis and colorectal tumorigenesis
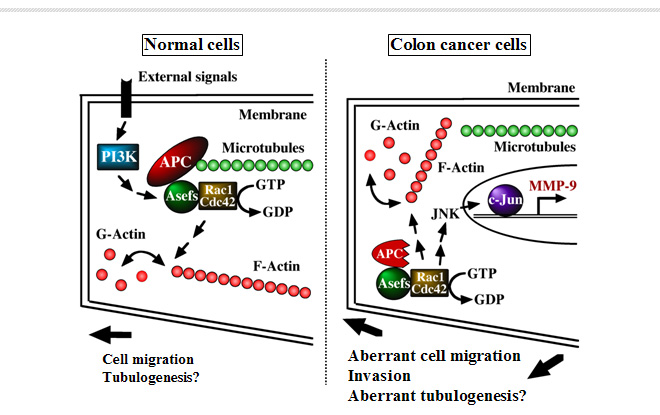
Purpose of the Research ProjectMutations of the tumor suppressor adenomatous polyposis coli (APC) are responsible for sporadic and familial colorectal tumors. APC negatively regulates Wnt signaling by inducing b-catenin degradation. It has also been shown that APC plays a role in the organization of cytoskeletal networks. APC interacts with Asef, Rac1- and Cdc42-specific guanine nucleotide exchange factors (GEFs), and stimulates their GEF activity; thereby regulating cell morphology, adhesion, and migration. Truncated mutant APCs present in colorectal tumor cells activate Asef constitutively and contribute to their aberrant migratory properties. Deficiency of either Asef significantly reduces the number and size of adenomas in ApcMin/+ mice, which are heterozygous for an APC mutation and spontaneously develop adenomas in the intestine. In this project, we attempt to clarify the role of APC/Asef in epithelial tube formation and the molecular mechanism of its dysfunction in tumorigenesis. Content of the Research ProjectOur previous study revealed that Asef is required for bFGF- and VEGF-induced tube formation of vascular endothelial cells in vitro. To extend our earlier observations on tubulogenesis, we will perform studies focused on the role of APC/Asef on regulation of cell polarity, migration and tube formation of epithelial cells. In addition, we attempt to clarify the mechanism by which its disruption leads to tumorigenesis. Expected Research Achievements and Scientific SignificanceInactivation of the tumour suppressor APC by biallelic mutations is responsible for sporadic and familial colorectal tumors. Although it is widely accepted that the ability of APC to negatively regulate Wnt signaling is essential for its tumor suppressor function, most mutations in colorectal tumors occur in APC and only a small percentage of mutations occur in β-catenin. It is therefore possible that inactivation of the additional APC functions might also be important for colorectal tumorigenesis. In this regard, it is interesting that APC interacts with Asef other than β-catenin. Thus, our study focused on APC/Asef would contribute to provide a new aspect of molecular mechanisms underlying APC-initiated tumorigenesis. In addition, research achievement in this project would be useful for the development of novel therapeutic approach and agents in colorectal tumors. As Asef-deficient mice appear normal and have a lifespan comparable with that of wild-type mice, compounds targeting Asef would be expected to have few serious side effects.
|